Last week, Advanced Cell Technology (ACT) of Massachusetts, USA, made two important announcements regarding human embryonic stem (hES) cell-based therapies for the potential treatment of Stargardt's dystrophy and age-related macular degeneration, two devastating degenerative diseases leading to blindness (reported in BioNews 642).
It's not a surprise that the news caused quite a stir. In developed countries, nearly 30 percent of people over 75 suffer, to some extent, from age-related macular degeneration. Stargardt's macular dystrophy causes progressive loss of vision from an early age in a very similar way, and is inherited in an autosomal recessive way.
There is no effective treatment currently available for either of the diseases. In England and Wales, age-related macular degeneration is the most commonly recorded main cause of certifications for both blindness (57 percent) and partial sight (56 percent) (1). Beside health and social impacts resulting from sight loss, economic impacts are also enormous. According to the Royal National Institute of Blind in UK the tab runs nearly £5 billion a year (2).
One of the two press releases announced the first ever European clinical trial using hES cell-based therapy. On 20 January, a team led by Professor James Bainbridge at the Moorfields Eye Hospital, London, injected cells from the pigmented layer of the retina (known as RPE cells), derived from hES cells, into an eye of a patient with Stargardt's macular degeneration.
The second announced the early online publication in the Lancet of preliminary data demonstrating the safety of hES cell-derived RPE cells for the treatment of two patients with Stargardt's macular dystrophy and dry age-related macular degeneration (3). The patients were both the first in Phase I/II clinical trials at the Jules Stein Eye Institute at the University of California, Los Angeles. No adverse effects arose after the transplantation; in particular, there was no abnormal proliferation of the cells and no tumour formation. Some visual improvement was seen, but due to the design of this safety study this could not be attributed to the treatment for certain.
Does this really mean, as suggested by some overenthusiastic media headlines, that the blind will now be able to see? We would be very cautious in making such statements. It is crucial to realise that these are primarily safety studies. Setting unrealistic expectations and goals could seriously undermine the results and affect the future of both ACT and the therapeutic prospects of hES cells.
In October 2011, citing financial reasons, another US company, Geron, discontinued its clinical trial for the treatment of spinal cord injuries with hES cells. We doubt the field could recover from another such blow, and it would leave the landscape for stem cell-based therapy and regenerative medicine irreparably changed.
However, if the therapeutic approach does work, as we all hope it will, it could mark the beginning of a golden age for hES cells, celebrating their power and potential in regenerative medicine.
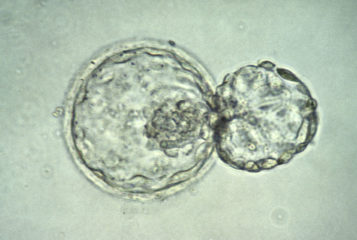
And that's not all. MA-09, the hES cell line used in this trial, was derived from a single biopsied cell (a blastomere) (4). Why is that different from hES cell lines derived from inner cell mass or a whole embryo? Blastomere biopsy of a third day embryo is a relatively harmless procedure done routinely for preimplantation genetic diagnostics. The remaining blastomeres are sufficient to develop into a fully competent blastocyst, leading to successful pregnancy.
MA-09 line was derived at the early stages of development and the embryo itself was destroyed in the process. Later, ACT and another company, StemLife Line, demonstrated that they could derive hES cell lines from biopsied blastomeres and still keep embryos alive (5). This proves the principle that successful hES cell-based therapy can be developed without embryo destruction. That would be a nail in the coffin of all naysayers.
Sources and References
-
3) The cost of sight loss in the UK: Campaign report 23, 2004.
-
4) Klimanskaya I, Chung Y, Becker S, Lu SJ, Lanza R. Human embryonic stem cell lines derived from single blastomeres
-
2) Bunce C, Wormald R. Leading causes of certification for blindness and partial sight in England and Wales
-
1) Schwartz SD, Hubschman JP, Heilwell G et al. Embryonic stem cell trials for macular degeneration: a preliminary report
-
5) Chung Y, Klimanskaya I, Becker S et al. Human embryonic stem cell lines generated without embryo destruction.



Leave a Reply
You must be logged in to post a comment.